What is the most humane way to kill crustaceans for human consumption?

Crustaceans show responses consistent with signs of pain and distress [1-5]. They also have the cognitive capacity to remember and learn to avoid unpleasant stimuli [6-8]. The RSPCA considers that, where crustaceans capable of experiencing pain and suffering are captured, handled, transported, stored and killed, that this must be done humanely. This applies to all decapod crustaceans, including crabs, crayfish, lobsters, Moreton Bay bugs, prawns, shrimps, and yabbies, whether the animal is to be eaten raw (sashimi) or cooked. Electrical stunning/killing is effective and humane.
The RSPCA recommends that live crustaceans are not made available for purchase by the public. Instead, they should be humanely killed by trained and competent personnel before purchase.
Humane treatment of crustaceans
A variety of methods are used to capture, hold, kill, and process crustaceans. The methods used depend on the species involved, the scale of the processing operation (commercial or non-commercial) and the end product. In each case, crustaceans should be killed by the most humane method.
Killing involves loss of sensibility (ability to feel pain), followed by death. For killing of crustaceans to be humane:
- the animal experiences an immediate loss of sensibility, or
- if loss of sensibility is not immediate, insensibility is induced without discomfort or pain.
Insensibility (unconsciousness) should persist until death ensues, without pain, suffering, or distress.
The legal status of crustaceans in Australia varies between different states and territories. In New South Wales, Victoria, the Northern Territory and the Australian Capital Territory, crustaceans are protected under the relevant animal welfare legislation (in some states, this only applies to crustaceans intended for human consumption). Penalties may apply if crustaceans are not treated humanely.
Skills and experience required
Humane killing requires skills and experience. Those competent to humanely kill crustaceans know how to:
- appropriately handle and care for live crustaceans to minimise stress and suffering
- apply the method of stunning to induce insensibility
- recognise signs of insensibility
- recognise signs of stress
- apply the method of killing
- apply a back-up stunning and/or killing method
- operate and maintain any equipment involved in the killing process.
Humane stunning
The purpose of stunning is to ensure the crustacean is unconscious and unable to experience pain, suffering, or distress prior to killing.
Electrical stunning using specialist equipment and the correct, species-specific electrical stunning specifications can effectively and humanely stun (within one second) decapod crustaceans (e.g., crabs, crayfish, lobsters, prawns/shrimps) [5, 9].
Only purpose-built electrical stunning equipment (e.g., the ‘Crustastun’ for lobsters, crabs, and crayfish) should be used, and in accordance with the manufacturer’s instructions. Failure to adequately electrically stun may have serious welfare consequences, including a high rate of autotomy (i.e., where the animal casts off of limbs or other body parts) [10].
Electrical stunning must be followed by a humane killing method unless the stun is sustained until the animal is dead.
Signs of insensibility
Signs of insensibility and therefore effective stunning, vary from species to species but generally include [10]:
- no resistance to handling – for example, the abdomen or tail can be easily manipulated, the outer mouth parts can be moved without resistance
- no control of limb movement
- no eye reactions when tapping on the shell
- no reaction when touching around the mouth parts.
Signs of stress
Signs of stress include [10]:
- thrashing
- autotomy (casting off of limbs or other body parts).
Humane killing
Following stunning, the unconscious animal must be immediately humanely killed. The RSPCA defines humane killing as when an animal is either killed instantly or rendered insensible until death ensues, without pain, suffering or distress.
For large decapod crustaceans (e.g., crabs and lobsters), acceptable methods are [5, 9]:
- electrical stunning followed by ‘spiking’ of crabs only
- electrical stunning followed by ‘splitting’ of lobsters and similar decapod crustaceans only
- electrical stunning followed by boiling.
Spiking
Spiking is suitable for stunned (i.e., unconscious) crabs. Crabs have two main nerve centres. One is located at the front of the animal, under a shallow depression. The other lies towards the rear of the animal and may have a small hole positioned over it (Figures 1 and 2). Crabs can be killed by rapid destruction (i.e., ‘double spiking’) of both nerve centres by piercing both ganglia from the underside of the crab with a pointed spike (e.g., a thick, pointed pithing instrument, an awl, or a sharp-pointed knife).
Spiking must not be performed on lobsters because they have a long chain of nerve centres which cannot be destroyed quickly through piercing.
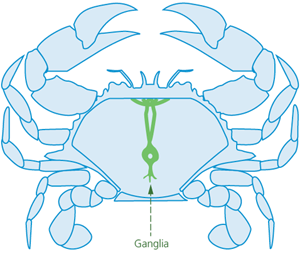
Figure 1 - Topside of crab, showing internal ganglia (nerve centres)
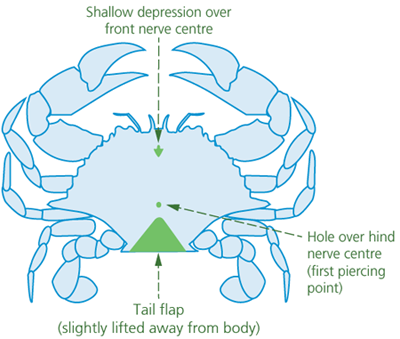
Figure 2 - Underside of crab, showing reference points for spiking
Splitting
Splitting is suitable for pre-stunned lobsters and similarly shaped species. Lobsters have a chain of nerve centres running down their central length (ventral longitudinal midline) (Figure 3). All the nerve centres are beneath the longitudinal midline on the animal’s undersurface, except the first nerve centre, the supraoesophageal ganglion, which is located at the top end of the nerve chain and is reached through the head.
Splitting involves rapidly cutting through the centre-line of the head, thorax (chest) and abdomen with a large, sharp knife. Cutting must occur along the longitudinal midline (lengthways) to destroy all the nerve centres (Figure 4).
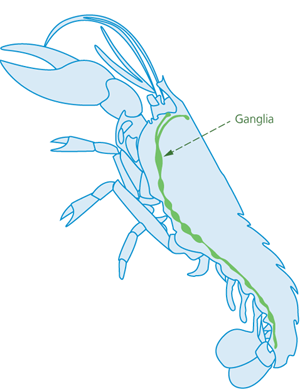
Figure 3 - Cross-section view of lobster, showing internal ganglia
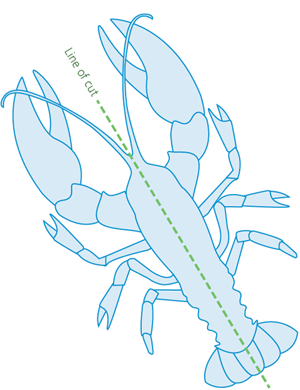
Figure 4 - Underside of lobster, showing line of cut for lobster splitting
Unacceptable stunning or killing methods
The following methods of stunning or killing crustaceans must not be used because they cause an unacceptable degree of pain and suffering to the animal [5, 9, 10]:
- Boiling (without prior stunning)
- Dismemberment
- Gassing (e.g., using carbon dioxide)
- Immersion in fresh water (osmotic shock)
- Immersion in water of high salinity.
Similarly, live crustaceans must not be:
- microwaved
- removed from the water and left to die from lack of oxygen
- placed in a container of water without adequate aeration and left to die from lack of oxygen
- exposed to caustic chemicals
- served in any dish involving a live crustacean for consumption.
Anaesthetic agents
Anaesthetic agents (e.g., AQUI-S clove oil) are not included as acceptable methods for the humane killing of crustaceans. Studies have not distinguished anaesthesia (and therefore insensibility) from paralysis and more research is needed to understand its mode of action [5]. There is no anaesthetic compound currently registered in Australia for use for the purpose of humanely killing crustaceans.
Chilling (dry or in ice)
Crustaceans are cold-blooded animals and enter a state of torpor when their body temperature is sufficiently reduced by chilling [5]. Once immobile, crustaceans are easier to handle and kill, which is likely why chilling is common. However, it is not well understood whether this immobility is also a sign of unconsciousness (i.e., insensibility) and indeed whether the chilling process (i.e., subjecting crustaceans to close to freezing temperatures) is in itself painful [5]. It is possible that chilling in dry air or in ice is painful and, once chilled, that it does not render the crustacean insensible to further pain from spiking, splitting, or cooking. Further research is needed to fully understand the effects of different chilling methods on crustacean welfare. Chilling of crustaceans is banned in Switzerland [5]. Adoption of electrical stunning technology for prawns is being promoted through the Shrimp Welfare Project’s Humane Slaughter Initiative as a method that is more effective and faster than chilling.
The way forward
Before being prepared for human consumption, decapod crustaceans are removed from their aquatic environment, then handled, transported, and stored. They may be wild caught (e.g., lobsters, crabs) or farmed (e.g., prawns). Regardless of origin, crustaceans are potentially exposed to multiple stressors prior to killing, including: exposure to air; inappropriate stocking densities, temperatures, and/or water quality during storage and transport; social stress and aggression when solitary decapods (e.g., lobsters) are held in the same tank; and, lack of access to dark environments and inability to avoid bright light [11]. Inappropriate stunning or killing methods will cause additional suffering.
Decapod crustaceans must be treated humanely – from capture through to killing – following best practice (see e.g., [9]) and a process of continual improvement that sees rapid uptake of alternative practices and/or new technology where the evidence demonstrates this will improve animal welfare.
References
Elwood R (2012) Evidence for pain in decapod crustaceans. Animal Welfare 21:23–27.
Barr S, Laming PR, Dick JTA, Elwood RW (2008) Nociception or pain in a decapod crustacean? Anim Behav 75(3):745–751.
Broom DM (2007) Cognitive ability and sentience: Which aquatic animals should be protected? Dis Aquat Organ 75:99–108.
Birch J, Burn C, Schnell A, Browning H, Crump A (2022) Review of the evidence of sentience in cephalopod molluscs and decapod crustaceans. Animal Welfare 31:155–156.
Elwood RW, Barr S, Patterson L (2009) Pain and stress in crustaceans? Appl Anim Behav Sci. 118:128–136.
Barr S, Elwood RW (2024) Trade-offs between avoidance of noxious electric shock and avoidance of bright light in shore crabs are consistent with predictions of pain. Animals 14(5):770.
Barr S, Elwood RW (2024) The effects of caustic soda and benzocaine on directed grooming to the eyestalk in the Glass Prawn, Palaemon elegans, are consistent with the idea of pain in decapods. Animals 14(3):364.
Crustacean Compassion (2023) Code of practice for the welfare of decapod crustaceans in the food chain: from capture to killing.
Conte F, Voslarova E, Vecerek V, Elwood RW, Coluccio P, Pugliese M, Passantino A (2021) Humane slaughter of edible decapod crustaceans. Animals 11(4):1089.
Crustacean Compassion (2023) Sea-to-plate: The welfare journey of decapod crustaceans.
Was this article helpful?
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.


